![]()
Cells typically have to maintain their internal environment relatively constant in the face of a chemically different and frequently fluctuating external environment. The membrane surrounding the cell (plasma membrane) is the interface between the two environments and constitutes both a structural and functional cell boundary. Inside the cell are ``sub-environments'' such as mitochondria, chloroplasts, lysosomes etc. whose internal environments are different yet again. Therefore, the membrane's primary job is compartmentalisation or encapsulation. However, if the only thing that the membrane does is to insure a proper boundary between things, then how come 20-50% of all proteins are membrane proteins. It is the presence of a membrane capable of regulating the passage of ions and solutes around cells or their organelles that enables them to create these different environments.
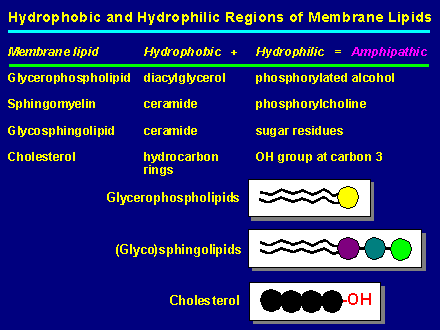
Eukaryotic and prokaryotic cell membranes are composed mainly of protein and lipid and a small but important amount of polysaccharide. Membrane lipids are all amphipathic (dual sympathy), i.e. they contain a hydrocarbon region which is hydrophobic (non polar) and a hydrophilic (polar) region which is usually smaller. Note that lipids are either neutral or negatively charged. Glycerophospholipids also known as phosphoglycerides (but normally called phospholipids just to confuse you), are the most abundant lipid components of most membranes. The tricarbon sugar glycerol is esterfied (or ether linked) at position 1 & 2 with fatty acids (diacylglycerol) and at position 3 with phosphate (phosphatidic acid). The phosphate in return is esterfied to a host of small compounds such as choline, glycerol, ethanolamine, inositol and others.
Phospholipids can spontaneously (i.e. it is thermodynamically favoured) assume 3 predominant forms in aqueous solution:
Which one of these will form depends on the type of phospholipid, the length and degree of saturation of the constituent fatty acids, the type of headgroup, the temperature, the ratio of lipid to the aqueous suspending medium and the mode of lipid dispersal. The different forms are all a result of the hydrophobic effect, the attempt to sequester non-polar hydrocarbon chains away from water. In micellar form the hydrocarbon chains are sequestered inside the micelle and the polar phospholipid head groups lie on the surface in contact with the water.
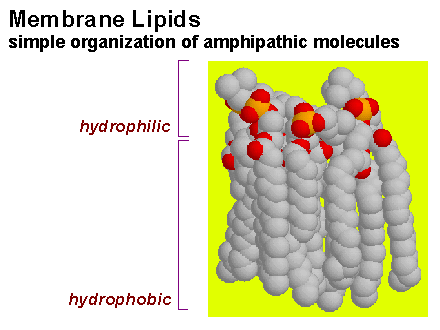
Most phospholipids also spontaneously form symmetrical sheet-like structures called bilayers. Bilayers are two molecules thick. The hydrocarbon chains of each layer minimize contact with water by joining together tail to tail in the centre of the bilayer. The close packing of these fatty acid chains is stabilised by van der Waals interactions between the hydrocarbon chain of adjacent phospholipids. Electrostatic and hydrogen bonds (as well as van der Waals interactions) stabilise the interaction of the polar phospholipid headgroups with water. Most of the common phospholipids are zwitterions, therefore there is no charge repulsion opposing close packing. The matching cross-sectional areas of these phospholipids (70 Å2) gives them a roughly cylindrical shape which tends to stabilise a bilayer structure. Note that there are no biological positively charged lipids, they are either negative or neutral. The physical properties of a lipid bilayer result in diffusion barrier for any ionic as well as larger polar (e.g. sugars) compounds. Note that small polar compounds such as water and alcohols will traverse the lipid bilayer.
Such a phospholipid bilayer could theoretically be of unlimited length and of unlimited thickness because individual bilayers can pack one on top of another (multilamellar bilayers). One thermodynamic problem you may have spotted concerning bilayers is that the hydrocarbon chains are exposed to the water at the ends of each sheet. To avoid this the bilayer will spontaneously round up and bring its exposed edges together to form a closed bilayer vesicle. The term liposome is used to denote a vesicle made up in the lab from synthetic lipids.
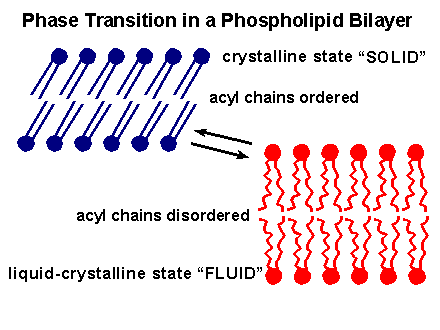
At very low temperatures the lipid bilayer will ``freeze'' and become a solid gel as the hydrocarbon chains take up a highly ordered close-packed conformation. At higher temperatures the forces holding the bilayer together are disrupted and the bilayer is replaced by a true disordered liquid solution consisting of individual lipid molecules.
Considerable evidence exists to show that, although it retains a bilayer structure, the close-packed gel-like state of the hydrocarbon phase that is formed at the low temperatures is incompatible with normal membrane function. Instead the membrane interior needs to be in a physical state intermediate between the frozen solid gel and a true molten liquid, the so called liquid crystal form.
Among the experimental evidence pointing to the importance of this liquid crystal state is the fact that organisms that cannot regulate their temperature by thermogenesis, e.g. plants, bacteria and some animals (poikilotherms) have evolved metabolic strategies to defend themselves against this threat of ``frostbite''.
Organisms like man which can maintain a constant body temperature even in a Fenland winter (homeotherms) are theoretically protected. However in unprotected parts of our bodies on chilly days we can occasionally experience the effect of our membranes becoming less fluid. The temperature in our fingers and toes can fall to a point where the membranes of the sensory nerve endings cease to function and we feel numb in those regions.
At the other extreme as you would probably predict, abnormally high temperatures can make a bilayer so fluid that it no longer retains it functions as a permeability barrier. Most poikilotherms are paralysed by temperatures above 43C probably because membranes in general and nerve membranes in particular become too ``leaky''.
The physical nature of this liquid crystal form can best be understood if we follow what happens when a frozen lipid is heated: As we heat up the lipid bilayer from the frozen gel-like close-packed form, a temperature is reached (transition temperature) at which there is an abrupt change in the physical properties of the bilayer.
In very simple terms, at the transition temperature the fatty acid side chains ``melt''. The effect is that the interior of the bilayer changes from a gel to a liquid, but since the phospholipid headgroups don't ``melt'' at this temperature, the overall bilayer structure is preserved.
This form of the bilayer is viewed as a 2D Dimensional fluid and is the preferred form for cellular membranes and cells have evolved mechanisms for regulating membrane fluidity by metabolically altering membrane lipid fatty acids. The reason for adopting this strategy is because the temperature at which the lipid interior of a given membrane is in the preferred liquid crystal state is determined by nature of the fatty acid hydrocarbon chains. Thus lipids containing fatty acids with shorter carbon chains (low melting point) undergo the phase transition at lower temperatures than lipids with long carbon chains (higher melting point).
Furthermore the melting points of saturated and unsaturated fatty acid chains are considerably different: membranes containing many saturated chains ``melt'' (undergo the gel to fluid phase transition) between 40C and 50C, whereas membranes consisting primarily of unsaturated chains melts near 0C or lower. The reason for this difference is that unsaturated fatty acids are ``kinky'', the presence of the cis double bond in an unsaturated fatty acid introduces a kink in the chain which means they cannot pack so tightly with neighbouring lipids and consequently form less stable van Der Waals contacts. Saturated chains fit together snugly and can optimise their van Der Waals interactions. Most naturally occurring fatty acids are cis .
Shape of fatty acids
Plants, animals and bacteria adapt to decreasing temperature by increasing the proportion of unsaturated fatty acids in the membrane, which tends to maintain a fluid bilayer at the reduced temperature. Conversely they respond to a rise in temperature by decreasing the proportion of unsaturated fatty acids and increasing the saturated fatty acid content. This is known as homeoviscous adaptation.
In eukaryote cells membrane cholesterol is also a major determinant of membrane fluidity. Cholesterol intercalates between phospholipids in the bilayer. Its polar hydroxyl group is in contact with the aqueous solution near the polar headgroups while the hydrophobic steroid ring interacts with and tends to immobilise the hydrocarbon chains. The effect of cholesterol one membrane fluidity varies depending on the temperature and on the lipid composition. Cholesterol restricts the random movement of the part of the hydrocarbon chains closest to the headgroups, but it separates the tails of the fatty acids and causes the inner regions of the bilayer to become more fluid.
Membrane proteins
Membrane proteins perform a huge variety of functions in the cell. Furthermore, understanding the function of membrane proteins is crucial not only for understanding normal cellular function but for better understanding most pharmacological agents. Remember that membrane proteins are the targets of most pharmacological agents. In fact, it has been estimated that a only one membrane protein family, the G-protein coupled receptors, serves as a target for more than 70% of all drugs. A few of the functions of membrane proteins are listed below:
1. Ion channels for rapid movement of ions (e.g. cystic fibrous Cl- channel) according to their electrochemical potential. Ion movement through channels are on the order of 107 ions/sec, which is between 1/2 and 1/3 of what is expected by free diffusion. Most ion channels are very large proteins consisting of repetitive units, for example the Ca2+ channel in the sacroplasmic reticulum (ryonodine receptor) is a tetrameric assembly of proteins, each more than 4000 amino-acids. Ions channels are responsible for transporting electrical signals as will be further elaborated in physiology lectures.
2. Transporters (or permeases) for specific compounds (e.g. amino-acids and sugars) according to their electrochemical gradient. Transporters can be specific or capable of transporting a multitude of compounds. In transporters the rate of movement across the membrane is markedly slower than channels, with rates ranging from 101-105.
3. Pumps transport a large variety of compounds from ions to proteins against their electrochemical gradient. Pumps like transporters, are usually specific for a particular compound, but non-specific transporters exist (e.g. multi drug transporters important in cancer and antibiotic resistance). The energy for transport may be either ATP or co-transport of an agent (e.g. H+ and Na+) down its electrochemical gradient. If the transport is in the same direction it is called symport, otherwise it is termed antiport. It has been estimated that one of these pumps the Na+/K+ ATPase is responsible for more than 70 % of our energetic expenditure alone in some tissues. Transport rates of pumps resemble that of the slower transporters.
4. Electron transport (including photosynthesis) takes place in the membranes of eukaryotic mitochondria, chloroplasts, bacteria, etc. The membrane provides a physical anchoring for the different players as well as an insulating layer preventing electrons and protons from free movement.
5. Additional enzymes reside in the membrane, amongst which we can note kinases (see next lecture) enzymes required for lipid synthesis and metabolism and many many others.
6. Receptors for hormones, neurotransmitters, and other chemicals are located in our membranes. The variation is astounding. As an example note that there are over 1000 different olfactory receptors alone.
7. Recognition and immunity is governed in part by membrane proteins (also glycolipids), including antigen presentation and cellular recognition.
There are many more activities taking place in the membrane, which space nor time allow us to consider at present.
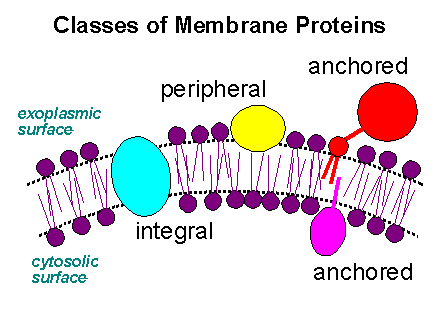
PROTEINS ARE ATTACHED TO MEMBRANES BY DIFFERENT MECHANISMS
1. Integral.
(a) lipid anchored.
i. GPI (and other) lipidation.
ii. Fatty acylation.
iii. Isoprenylation.
(b) Self anchored.
i. Single transmembrane -helix (monotopic).
ii. Multiple transmembrane -helices (polytopic).
iii. Beta-barrel.
2. Peripheral.
3. Transient.
The operative distinction between integral and peripheral membrane proteins is that integral membrane proteins can be disassociated from the bilayer only with agents that demolish bilayer integrity (e.g. detergents and organic solvents). Peripheral membrane proteins are normally attached to the bilayer via electrostatic interaction (with the lipid charged headgroups) and can therefore be removed using high salt washes. Note that it is very difficult to delineate between cytoplasmic proteins and peripheral membrane proteins because the cytoskeleton is membrane associated and therefore proteins that associate with the cytoskeleton (as many protein do) are consequently associated with the membrane.
Integral membrane proteins are attached to the membrane (anchored) via a hydrophobic element that is inserted in the bilayer. The hydrophobic element that is inserted in the bilayer can either be part of the protein chain (self anchored) or a result of covalently attached lipid group (lipid anchored). Note that a proteins can be both lipid anchored and self anchored. The reason for this ``redundancy'' is not clear.
Lipid anchored
Three common types of lipid anchoring are found, which need not be mutually exclusive.
1. Covalent attachment to phosphatidyl inositol (PI anchored). Interestingly, many proteins that are PI anchored start off as being self anchored (see below) but are then cleaved from this transmembrane element and transferred to PI. The reason for this reaction is unclear (perhaps an increase in the diffusion constant). Other attachments are found such as a thioether linkage in the murin lipoprotein in the outer membrane of gram negative bacteria. 2. Covalent thioester (cysteine) or ester (threonine) linkage to fatty acids, mostly palmitic, myristic, stearic and oleic acids. Myristic acid is also found amidated to the N-terminus of proteins. 3. Thioether linkage to isoprenyl group, normally farnesyl and geranylgeranyl, 3 and 4 isoprene units, respectively.
Self anchoring
A great number of membrane proteins span the bilayer completely. Notice that they are therefore in contact both with the inside and the outside of the cell and ideally situated, as we shall see in more detail later, to relay signals from one side to the other.
Transmembrane elements of proteins reside in a hydrophobic enviornment, where no hydrogen bonding groups are present, therefore the protein must satisfy its own hydrogen-bonding potential. The two most common way of satisfying their own hydrogen-bonding potential is by forming an -helix (and helical bundles) or a -barrel. As you would expect the -helices and -sheets that reside in the membrane would therefore, be much more stable than their water soluble counterparts.