![]()
![]()
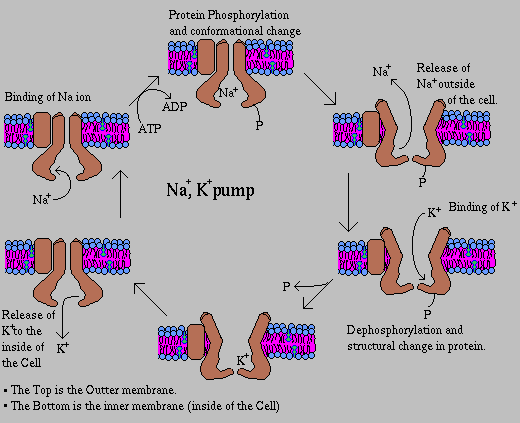
Most animal cells have a high concentration of K+ and a low concentration of Na+ relative to the external medium. These ionic gradients are generated by a specific transport system that is called the Na+,K+ pump because the movement of these ions is linked. The active transport of Na+ and K+ is of great physiological significance. The Na+-K+ gradient in animal cells control cell volume, renders nerve and muscle cells electrically excitable, and drives the active transport of sugars and amino acids.
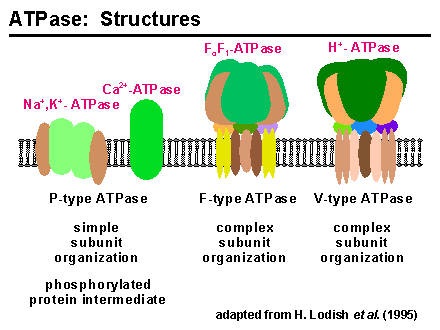
In 1957, Jens Skou discovered an enzyme that hydrolyzes ATP only if Na+ and K+ are present in addition Mg2+. This enzyme is called a Na+,K+-ATPase.
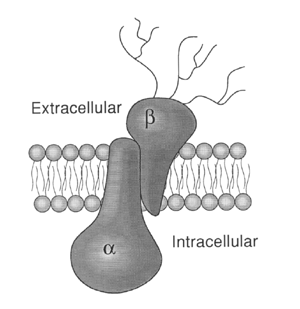
The pump consists of two dissimilar subunits: a 110,000-dalton alpha subunit and a 55,000-dalton beta subunit. The subunits are present in equimolar amounts, and the likely structure of the enzyme complex is an (alpha-beta)2 dimer. The alpha subunit contains the binding sites for the substrates required by the enzyme and exists as a phosphorylated intermediate during the catalytic cycle. The role of the beta subunit is unknown, although it is necessary for normal pump function and may play a role in stabilizing alpha.